Effects Of Heavy Metals On Human Health Pdf
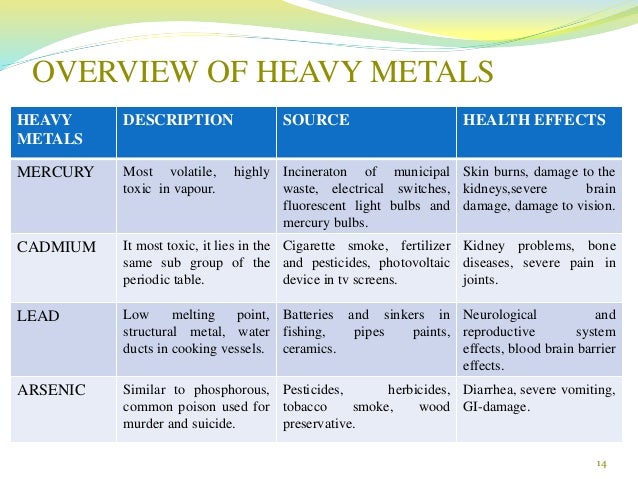
Heavy metals are thus commonly defined as those having a specific density of more than 5 g/cm 3. The main threats to human health from heavy metals are associated with exposure to lead, cadmium, mercury and arsenic (arsenic is a metalloid, but is usually classified as a heavy metal). Everyday, the body is exposed to toxins and heavy metals. Here are common metals with documented toxicities and varying risk of unintentional overexposure. The Effects of Heavy Metals on. Formation constitute a dangerous problem for human health. Metals causes contamination of fish with these metals. The effects of.
Most heavy metals are necessary trace elements for human body.However the heavy metal pollution has become a key environmental problem endangering the development of humankind.Excessive heavy metal poses great danger to human body by presenting strong propagation resistance,obstructing normal embryonic development and effecting the normal growth of children.It seriously restricts the human health and the sustainable development,which compose of the most important part of the sustainable development of eco-system.
Abstract The main threats to human health from heavy metals are associated with exposure to lead, cadmium, mercury and arsenic. These metals have been extensively studied and their effects on human health regularly reviewed by international bodies such as the WHO. Heavy metals have been used by humans for thousands of years. Although several adverse health effects of heavy metals have been known for a long time, exposure to heavy metals continues, and is even increasing in some parts of the world, in particular in less developed countries, though emissions have declined in most developed countries over the last 100 years. Cadmium compounds are currently mainly used in re-chargeable nickel–cadmium batteries.
Cadmium emissions have increased dramatically during the 20th century, one reason being that cadmium-containing products are rarely re-cycled, but often dumped together with household waste. Cigarette smoking is a major source of cadmium exposure. In non-smokers, food is the most important source of cadmium exposure.
Recent data indicate that adverse health effects of cadmium exposure may occur at lower exposure levels than previously anticipated, primarily in the form of kidney damage but possibly also bone effects and fractures. Many individuals in Europe already exceed these exposure levels and the margin is very narrow for large groups.
Therefore, measures should be taken to reduce cadmium exposure in the general population in order to minimize the risk of adverse health effects. The general population is primarily exposed to mercury via food, fish being a major source of methyl mercury exposure, and dental amalgam. The general population does not face a significant health risk from methyl mercury, although certain groups with high fish consumption may attain blood levels associated with a low risk of neurological damage to adults. Since there is a risk to the fetus in particular, pregnant women should avoid a high intake of certain fish, such as shark, swordfish and tuna; fish (such as pike, walleye and bass) taken from polluted fresh waters should especially be avoided. There has been a debate on the safety of dental amalgams and claims have been made that mercury from amalgam may cause a variety of diseases. However, there are no studies so far that have been able to show any associations between amalgam fillings and ill health. The general population is exposed to lead from air and food in roughly equal proportions.
During the last century, lead emissions to ambient air have caused considerable pollution, mainly due to lead emissions from petrol. Children are particularly susceptible to lead exposure due to high gastrointestinal uptake and the permeable blood–brain barrier. Blood levels in children should be reduced below the levels so far considered acceptable, recent data indicating that there may be neurotoxic effects of lead at lower levels of exposure than previously anticipated. Although lead in petrol has dramatically decreased over the last decades, thereby reducing environmental exposure, phasing out any remaining uses of lead additives in motor fuels should be encouraged.
The use of lead-based paints should be abandoned, and lead should not be used in food containers. In particular, the public should be aware of glazed food containers, which may leach lead into food.
Exposure to arsenic is mainly via intake of food and drinking water, food being the most important source in most populations. Long-term exposure to arsenic in drinking-water is mainly related to increased risks of skin cancer, but also some other cancers, as well as other skin lesions such as hyperkeratosis and pigmentation changes. Occupational exposure to arsenic, primarily by inhalation, is causally associated with lung cancer.
Clear exposure–response relationships and high risks have been observed. Introduction Although there is no clear definition of what a heavy metal is, density is in most cases taken to be the defining factor. Heavy metals are thus commonly defined as those having a specific density of more than 5 g/cm 3. The main threats to human health from heavy metals are associated with exposure to lead, cadmium, mercury and arsenic (arsenic is a metalloid, but is usually classified as a heavy metal).
Heavy metals have been used in many different areas for thousands of years. Lead has been used for at least 5000 years, early applications including building materials, pigments for glazing ceramics, and pipes for transporting water. In ancient Rome, lead acetate was used to sweeten old wine, and some Romans might have consumed as much as a gram of lead a day. Mercury was allegedly used by the Romans as a salve to alleviate teething pain in infants, and was later (from the 1300s to the late 1800s) employed as a remedy for syphilis. Claude Monet used cadmium pigments extensively in the mid 1800s, but the scarcity of the metal limited the use in artists’ materials until the early 1900s.
Although adverse health effects of heavy metals have been known for a long time, exposure to heavy metals continues and is even increasing in some areas. For example, mercury is still used in gold mining in many parts of Latin America. Arsenic is still common in wood preservatives, and tetraethyl lead remains a common additive to petrol, although this use has decreased dramatically in the developed countries. Since the middle of the 19th century, production of heavy metals increased steeply for more than 100 years, with concomitant emissions to the environment (Fig.
Global production and consumption of selected toxic metals, 1850–1990. At the end of the 20th century, however, emissions of heavy metals started to decrease in developed countries: in the UK, emissions of heavy metals fell by over 50% between 1990 and 2000.
Emissions of heavy metals to the environment occur via a wide range of processes and pathways, including to the air ( e.g. During combustion, extraction and processing), to surface waters ( via runoff and releases from storage and transport) and to the soil (and hence into groundwaters and crops) (see Chapter 1). Atmospheric emissions tend to be of greatest concern in terms of human health, both because of the quantities involved and the widespread dispersion and potential for exposure that often ensues. The spatial distributions of cadmium, lead and mercury emissions to the atmosphere in Europe can be found in the Meteorological Synthesizing Centre-East (MSC-E) website. Lead emissions are mainly related to road transport and thus most uniformly distributed over space. Cadmium emissions are primarily associated with non-ferrous metallurgy and fuel combustion, whereas the spatial distribution of anthropogenic mercury emissions reflects mainly the level of coal consumption in different regions.
People may be exposed to potentially harmful chemical, physical and biological agents in air, food, water or soil. However, exposure does not result only from the presence of a harmful agent in the environment.
The key word in the definition of exposure is contact. There must be contact between the agent and the outer boundary of the human body, such as the airways, the skin or the mouth. Exposure is often defined as a function of concentration and time: “an event that occurs when there is contact at a boundary between a human and the environment with a contaminant of a specific concentration for an interval of time”. For exposure to happen, therefore, co-existence of heavy metals and people has to occur (see Chapter 1). Cadmium Occurrence, exposure and dose Cadmium occurs naturally in ores together with zinc, lead and copper. Cadmium compounds are used as stabilizers in PVC products, colour pigment, several alloys and, now most commonly, in re-chargeable nickel–cadmium batteries. Metallic cadmium has mostly been used as an anticorrosion agent (cadmiation).
Cadmium is also present as a pollutant in phosphate fertilizers. EU cadmium usage has decreased considerably during the 1990s, mainly due to the gradual phase-out of cadmium products other than Ni-Cd batteries and the implementation of more stringent EU environmental legislation (Directive 91/338/ECC). Notwithstanding these reductions in Europe, however, cadmium production, consumption and emissions to the environment worldwide have increased dramatically during the 20th century.
Cadmium containing products are rarely re-cycled, but frequently dumped together with household waste, thereby contaminating the environment, especially if the waste is incinerated. Natural as well as anthropogenic sources of cadmium, including industrial emissions and the application of fertilizer and sewage sludge to farm land, may lead to contamination of soils, and to increased cadmium uptake by crops and vegetables, grown for human consumption. The uptake process of soil cadmium by plants is enhanced at low pH. Cigarette smoking is a major source of cadmium exposure. Biological monitoring of cadmium in the general population has shown that cigarette smoking may cause significant increases in blood cadmium (B-Cd) levels, the concentrations in smokers being on average 4–5 times higher than those in non-smokers. Despite evidence of exposure from environmental tobacco smoke, however, this is probably contributing little to total cadmium body burden. Food is the most important source of cadmium exposure in the general non-smoking population in most countries.
Varsha Mudgal
Cadmium is present in most foodstuffs, but concentrations vary greatly, and individual intake also varies considerably due to differences in dietary habits. Double bass concertos. Women usually have lower daily cadmium intakes, because of lower energy consumption than men.
Gastrointestinal absorption of cadmium may be influenced by nutritional factors, such as iron status. B-Cd generally reflects current exposure, but partly also lifetime body burden. The cadmium concentration in urine (U-Cd) is mainly influenced by the body burden, U-Cd being proportional to the kidney concentration. Smokers and people living in contaminated areas have higher urinary cadmium concentrations, smokers having about twice as high concentrations as non-smokers. Health effects Inhalation of cadmium fumes or particles can be life threatening, and although acute pulmonary effects and deaths are uncommon, sporadic cases still occur. Cadmium exposure may cause kidney damage.
The first sign of the renal lesion is usually a tubular dysfunction, evidenced by an increased excretion of low molecular weight proteins such as β 2-microglobulin and α 1-microglobulin (protein HC) or enzymes such as N-Acetyl-β-D-glucosaminidase (NAG). It has been suggested that the tubular damage is reversible, but there is overwhelming evidence that the cadmium induced tubular damage is indeed irreversible. WHO estimated that a urinary excretion of 10 nmol/mmol creatinine (corresponding to circa 200 mg Cd/kg kidney cortex) would constitute a ‘critical limit’ below which kidney damage would not occur. However, WHO calculated that circa 10% of individuals with this kidney concentration would be affected by tubular damage.
Several reports have since shown that kidney damage and/or bone effects are likely to occur at lower kidney cadmium levels. European studies have shown signs of cadmium induced kidney damage in the general population at urinary cadmium levels around 2–3 μg Cd/g creatinine. The initial tubular damage may progress to more severe kidney damage, and already in 1950 it was reported that some cadmium exposed workers had developed decreased glomerular filtration rate (GFR). This has been confirmed in later studies of occupationally exposed workers. An excess risk of kidney stones, possibly related to an increased excretion of calcium in urine following the tubular damage, has been shown in several studies. Recently, an association between cadmium exposure and chronic renal failure end stage renal disease (ESRD) was shown.
Using a registry of patients, who had been treated for uraemia, the investigators found a double risk of ESRD in persons living close to (. Lead concentrations in petrol and children’s blood (USA).
Source: redrawn from Annest (1983), as reproduced in National Academy of Sciences/National Research Council. Measuring Lead Exposure in Infants, Children, and Other Sensitive Populations. Washington, DC, USA: National Academy Press, 1993. Occupational exposure to inorganic lead occurs in mines and smelters as well as welding of lead painted metal, and in battery plants. Low or moderate exposure may take place in the glass industry. High levels of air emissions may pollute areas near lead mines and smelters. Airborne lead can be deposited on soil and water, thus reaching humans via the food chain.
Up to 50% of inhaled inorganic lead may be absorbed in the lungs. Adults take up 10–15% of lead in food, whereas children may absorb up to 50% via the gastrointestinal tract. Lead in blood is bound to erythrocytes, and elimination is slow and principally via urine.
Lead is accumulated in the skeleton, and is only slowly released from this body compartment. Half-life of lead in blood is about 1 month and in the skeleton 20–30 years. In adults, inorganic lead does not penetrate the blood–brain barrier, whereas this barrier is less developed in children. The high gastrointestinal uptake and the permeable blood–brain barrier make children especially susceptible to lead exposure and subsequent brain damage.
Organic lead compounds penetrate body and cell membranes. Tetramethyl lead and tetraethyl lead penetrate the skin easily. These compounds may also cross the blood–brain barrier in adults, and thus adults may suffer from lead encephalopathy related to acute poisoning by organic lead compounds. Health effects The symptoms of acute lead poisoning are headache, irritability, abdominal pain and various symptoms related to the nervous system. Lead encephalopathy is characterized by sleeplessness and restlessness.
Children may be affected by behavioural disturbances, learning and concentration difficulties. In severe cases of lead encephalopathy, the affected person may suffer from acute psychosis, confusion and reduced consciousness. People who have been exposed to lead for a long time may suffer from memory deterioration, prolonged reaction time and reduced ability to understand. Individuals with average blood lead levels under 3 μmol/l may show signs of peripheral nerve symptoms with reduced nerve conduction velocity and reduced dermal sensibility. If the neuropathy is severe the lesion may be permanent.
The classical picture includes a dark blue lead sulphide line at the gingival margin. In less serious cases, the most obvious sign of lead poisoning is disturbance of haemoglobin synthesis, and long-term lead exposure may lead to anaemia. Recent research has shown that long-term low-level lead exposure in children may also lead to diminished intellectual capacity. Figure shows a meta-analysis of four prospective studies using mean blood lead level over a number of years. The combined evidence suggests a weighted mean decrease in IQ of 2 points for a 0.48 μmol/l (10 μg/dl) increase in blood lead level (95% confidence interval from −0.3 points to −3.6 points).
Estimated mean change in IQ for an increase in blood lead level from 0.48 to 0.96 μmol/l (10–20 μg/dl) from a meta-analysis of four prospective studies. Acute exposure to lead is known to cause proximal renal tubular damage. Long-term lead exposure may also give rise to kidney damage and, in a recent study of Egyptian policemen, urinary excretion of NAG was positively correlated with duration of exposure to lead from automobile exhaust, blood lead and nail lead. Despite intensive efforts to define the relationship between body burden of lead and blood pressure or other effects on the cardiovascular system, no causal relationship has been demonstrated in humans.
Using routinely collected data on mortality (1981–96), hospital episode statistics data 1992–1995 and statutory returns to the Health and Safety Executive (RIDDOR), one death and 83 hospital cases were identified. The authors found that mortality and hospital admission ascribed to lead poisoning in England were rare, but that cases continue to occur and that some seem to be associated with considerable morbidity. Blood lead levels in children below 10 μmg/dl have so far been considered acceptable, but recent data indicate that there may be toxicological effects of lead at lower levels of exposure than previously anticipated. There is also evidence that certain genetic and environmental factors can increase the detrimental effects of lead on neural development, thereby rendering certain children more vulnerable to lead neurotoxicity. IARC classified lead as a ‘possible human carcinogen’ based on sufficient animal data and insufficient human data in 1987.
Since then a few studies have been published, the overall evidence for lead as a carcinogen being only weak, the most likely candidates are lung cancer, stomach cancer and gliomas. Arsenic Occurrence, exposure and dose Arsenic is a widely distributed metalloid, occurring in rock, soil, water and air. Inorganic arsenic is present in groundwater used for drinking in several countries all over the world ( e.g. Bangladesh, Chile and China), whereas organic arsenic compounds (such as arsenobetaine) are primarily found in fish, which thus may give rise to human exposure. Smelting of non-ferrous metals and the production of energy from fossil fuel are the two major industrial processes that lead to arsenic contamination of air, water and soil, smelting activities being the largest single anthropogenic source of atmospheric pollution. Other sources of contamination are the manufacture and use of arsenical pesticides and wood preservatives.
Sanjay …
The working group of the EU DG Environment concluded that there were large reductions in the emissions of arsenic to air in several member countries of the European Union in the 1980s. In 1990, the total emissions of arsenic to the air in the member states were estimated to be 575 tonnes. In 1996, the estimated total releases of arsenic to the air in the UK were 50 tonnes. Concentrations in air in rural areas range from 1000 ng/m 3) have been measured near industrial sources. Water concentrations are usually.